Abstract
Background: Natural killer (NK) cells are an "off the shelf" cell-based therapy capable of inducing remissions and long-term survival in hematological malignancies. Despite responses seen with NK cellular therapy, most remissions are transient necessitating enhanced strategies for maintaining deeper responses. Pre-clinical studies show that aryl hydrocarbon receptor (AHR) inhibition restores a normal NK cell maturation profile in AML patient samples via miR-29b dependent pathway and sensitizes AML blasts to NK cell mediated killing. The AHR pathway also drives blast maturation, particularly monocytic differentiation through upregulation of CD14 and CD11b. Venetoclax resistance in AML patients is a major clinical barrier, characterized by the outgrowth of monocytic subclones with low BCL2 expression. HLA-E, an inhibitory ligand expressed on blasts, blocks NK cell function. IK-364, a compound generated by IKENA Oncology, is a potent and selective AHR inhibitor in preclinical investigation. Given the connection with monocytic maturation and enhanced NK cell function through AHR blockade, we hypothesized that AHR drives Venetoclax resistance and impaired NK cell function. We therefore investigated the role of AHR pathway activation in AML and functional consequence of blocking this pathway in AML patient samples.
Methods: Primary leukemic cells and healthy donor peripheral blood mononuclear cells (PBMCs) were obtained under IRB-approved protocols. Human cell lines were obtained from ATCC. NK cells were magnetically enriched and expanded on K562 -IL21 feeder cells. IK-364 was obtained from IKENA Oncology. Primary AML PBMCs, previously frozen, were thawed and placed in RPMI media containing 20% fetal bovine serum, 1% antibiotic/antimycotic, human interleukin-3 (hIL-3; 10ng/mL), hIL-6 (100 ng/mL) and stem cell factor (100 ng/mL). Blasts were co-cultured with IK-364 at 3uM or DMSO (vehicle control) for 48 hours. NK cell cytotoxicity was measured by flow cytometric detection of caspase-3/7 and SytoxAAD activation. Immunophenotyping was conducted by flow cytometry.
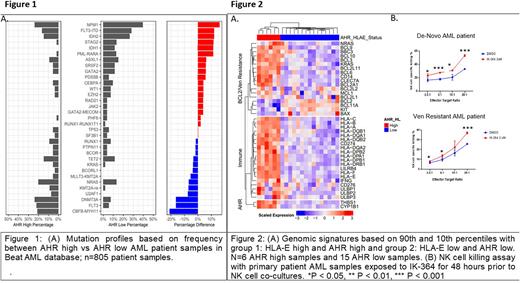
Results: We ranked the highest and lowest 10% expression levels of AHR by RNA expression (RPKM) across all samples in the Beat AML dataset and evaluated their mutation profiles. Ranking the mutations based on frequency we found an enrichment of mutations in NPM1, IDH2, IDH1, PML-RARA and FLT3-ITD in the low AHR expression group compared to the high expression group (Figure1A). AHR high patients had higher frequencies of CBF-MYH11, DNMT3A, FLT3 point mutations, NRAS, KRAS and BCOR mutations, compared to AHR low expressors. Immunophenotype analysis of AHR high samples shows more mature phenotypic markers HLA-DR, CD11b and HLA-E as well as more HLA Class I expression. Compared to AHR-low AML samples, AHR-high patients had altered NK cell profiles in the blood characterized by less mature stage 5 NK cells. High expression levels of AHR and HLA-E correlates with Venetoclax resistance including RAS pathway upregulation and differential expression of various BCL2 family members, such as downregulation of BCL2 (Figure 2A). Blocking AHR pathway with an AHR inhibitor, IK-364, resulted in downregulation of HLA-E and LILRB4 and enhanced NK cell killing in de-novo and Venetoclax resistant patient samples (Figure 2B).
Discussion: We demonstrate that AHR expression in AML correlates with a distinct genomic profile characterized by mature monocytic blast phenotype and immune resistance. Further analysis of patient samples shows a correlation between HLA-E expression and AHR pathway activation defined by blast differentiation state, and showed a correlation between Venetoclax resistance signatures in AHR-high samples. The AHR inhibitor, IK-364, downregulated multiple immune ligands including HLA-E and LILRB4 on FLT3 mutation positive patient samples and led to enhanced NK cell mediated cytotoxicity. We show that AHR-high samples have more HLA class I expression on mature blasts than immature, highlighting the importance of blast differentiation state in modulating treatment response. These data support continued development of AHR inhibition as a therapeutic strategy in combination with NK cells in Venetoclax-resistant AML.
Disclosures
Saultz:IKENA: Research Funding. McGovern:IKENA: Current Employment. Wang:IKENA: Current Employment. Sanchez-Martin:IKENA: Current Employment. Kaufman:Qihan Biotech: Consultancy; VisiCell: Consultancy; Shoreline Bioscience: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees, Research Funding. Tyner:Recludix Pharma: Membership on an entity's Board of Directors or advisory committees; Acerta: Research Funding; Agios: Research Funding; Aptose: Research Funding; Array: Research Funding; Astra-Zeneca: Research Funding; Constellation: Research Funding; Genentech: Research Funding; Gilead: Research Funding; Incyte: Research Funding; Janssen: Research Funding; Kronos: Research Funding; Meryx: Research Funding; Petra: Research Funding; Schrodinger: Research Funding; Seattle Genetics: Research Funding; Syros: Research Funding; Takeda: Research Funding; Tolero: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal